Research Article
The Effect of Different forms of Application of Chlorhexidine Gluconate on Clinical Parameters and Patients with Periodontal Disease
- Sahmedin Saliu *
Department of Periodontology, Faculty of Dentistry, University of Tetovo, Macedonia.
*Corresponding Author: Sahmedin Saliu, Department of Periodontology, Faculty of Dentistry, University of Tetovo, Macedonia.
Citation: Saliu S., Fidoski J. (2024). The Effect of Different Forms of Application of Chlorhexidine Gluconate on Clinical Parameters and Patients with Periodontal Disease, Clinical Case Reports and Studies, BioRes Scientia Publishers. 7(6):1-11. DOI: 10.59657/2837-2565.brs.24.202
Copyright: © 2024 Sahmedin Saliu, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: November 06, 2024 | Accepted: November 20, 2024 | Published: December 01, 2024
Abstract
Introduction: To compare the clinical effects of two periodontal treatment modalities: application of 0.12 % chlorhexidine digluconate solution versus application of PerioChip after scaling and root planning (SRP) in patients suffering from periodontal disease.
Methods: 30 periodontitis patients with at least two bilateral periodontal pockets with PD≥ 5 mm were recruited for this split mouth study. The selected sites were then treated with SRP plus subgingival irrigation of 0.12 % chlorhexidine digluconate solution or SRP plus subgingival application of PerioChip. Index of gingival inflammation (GI) as well as dental plaque index (PI) were recorded 15, 30 and 90 days after treatment. The concentration of chlorhexidine digluconate in GCF was determined using liquid chromatography.
Results: Overall, the clinical parameters after three months were better in the group treated with SRP plus subgingival insertion of the PerioChip. The concentrations of chlorhexidine in GCF were highest one hour after the application of the PerioChip and remained above 125 µg mL-1 seven days after the beginning of the treatment. The application of 0.12 % chlorhexidine digluconate solution in the selected sites resulted in concentrations below the limit of detection.
Conclusion: Adjunctive use of slow-release chlorhexidine formulations such as PerioChip might be considered in the management of periodontal disease and gingival inflammation to reduce the need of further therapeutic procedures.
Keywords: chronic periodontal disease, chlorhexidine digluconate, periochip, gingival crevicular fluid, periodontal therapy
Introduction
Periodontitis, a plaque-induced chronic inflammatory disease, affects the supporting structures of the periodontium, leading to progressive destruction of connective tissue attachment and alveolar bone loss. The disease is initiated by microorganisms residing in the subgingival biofilm that require a susceptible host to elicit the chronic inflammatory reaction responsible of the tissue destruction [1]. Bacterial biofilms are a complex aggregation of microorganisms growing on a solid substrate. They contain more than 500 microorganisms which release toxins as well as enzymes and cause progressive tissue destruction and alveolar bone loss [2]. Nowadays, the main focus of the therapeutic interventions in the treatment and prevention of the disease is still based on the control of the bacterial biofilm [2,3]. The gold standard in the treatment of periodontitis is mechanical debridement of periodontal pockets by scaling and root planning (SRP), thus aiming at the elimination or disruption of the subgingival biofilm. This procedure, in conjunction with regular daily oral hygiene regimen, has shown highly effective in the prevention and treatment of most periodontal diseases [3]. In addition to the mechanical treatment, the use of antimicrobial agents, both systemic and topical, has increased due to the fact that periodontal disease is not merely an overgrowth of bacteria, but also a shift in bacterial species [4].
Antibiotics and antiseptics have been used successfully to treat moderate-to-severe periodontal disease [5]. The local application of antimicrobials has shown a clear advantage in cases where localized pockets are present or in the treatment of non-responding and recurrent sites. In these cases, locally applied antimicrobial agents lack the adverse effects associated with systemic medications and do not depend on the patient’s compliance [1]. However, for a locally applied antimicrobial agent to be effective in the treatment of deep or recurrent pockets, it must reach high concentrations in GCF and maintain these high levels during a period of at least 7 days [6,7]. Among the available antimicrobial agents applied locally in the treatment of periodontal disease, the antiseptic chlorhexidine (CHX) has clearly demonstrated its clinical efficacy8. It is very effective when used for subgingival irrigation in the form of 0.12 % solution, although there are some adverse effects such as staining of the teeth, oral mucosal erosion or bitter taste. Recent advances in periodontal drug delivery systems have led to the development of controlled-delivery systems for local application in the periodontal pockets. These systems maintain effective therapeutic concentrations for up to 10 days and their application results in favorable clinical outcome. The PerioChip is a small orange-brown, biodegradable rectangular chip, rounded at one edge which allows easier insertion in the periodontal pockets. It contains 2.5 mg of chlorhexidine digluconate in a biodegradable matrix of hydrolyzed gelatin cross-linked with glutaraldehide. It is used as adjunct to SRP in adult periodontitis patients and as a part of maintenance program which includes oral hygiene and regular scaling and root planning. Based on previous findings, the purpose of our study was to evaluate the therapeutic effect of 0.12 % CHX solution and PerioChip application on the clinical parameters in patients suffering from periodontal disease, when used as adjunct to SRP.
Materials and Methods
The present study was a randomized split-mouth study. The study protocol was submitted and approved by the Ethical Committee of the Faculty of Dentistry and the study was conducted between July and September 2013. 30 patients (17 men and 13 women), aged 40-65, were selected from patients receiving treatment at the Department of Periodontology, Faculty of Dentistry, Skopje. The patients were in good general health and have been diagnosed clinically and radiographically with chronic periodontitis with at least two bilateral periodontal pockets with pocket depth PD>5 mm. At the selected sites, the index of gingival inflammation and dental plaque were recorded [9,10], as baseline data. One of the selected areas received SRP followed by application of 0.12 % CHX solution, and the other selected tooth received SRP followed by insertion of the PerioChip. Recordings of the clinical parameters were taken 15, 30 and 90 days after the beginning of the treatment. All the patients received written consent prior to the inclusion in the study. Exclusion criteria included: oral mucosal-inflammatory lesions, antibiotic/anti-inflammatory therapy at least three months prior baseline examinations, pregnancy, history of hypersensitivity to CHX and smoking. For quantitative determination of CHX concentrations in GCF samples after the treatment, samples were taken after 1, 24, 48, 96 and 168 hours and kept frozen at – 20 ° C until analysis.
Collection of GCF Samples
GCF samples were obtained from 30 patients suffering from chronic periodontitis, with probing pocket depths of ≥ 5 mm. A single chip was placed into each predetermined periodontal pocket using dental forceps, after isolating and drying the associated tooth. The PerioChip completely decomposes 7-10 days after placement and therefore GCF samples were collected after 1 and 24 hours and after 2, 4, 6 and 7 days after Chip placement. Another GCF sample was taken immediately after subgingival irrigation with 0.12 % CHX solution. GCF was collected applying the method of Koss et al [11].
RP-HPLC Determination of CHX Concentrations in GCF
For routine analysis of CHX concentrations in GCF a simple and rapid bioanalytical method was developed and validated. The chromatographic analysis was conducted on Shimadzu Nexera system equipped with UV diode array detector. The chromatographic separation was performed on Discovery C18, 250 x 4.6 mm, 5µm (Supelco, USA) at 25 °C. Chlorpheniramine was used a as an internal standard (IS). The mobile phase consisted of acetonitrile, 0.01 mol L-1 phosphate buffer (pH=3) and triethylamine (33:66:1, V/V/V). Flow rate was 1 ml min-1. The injection volume was 50 µL and the UV detection was performed at 253 nm. The total run time for the HPLC analysis was 10 min.
GCF Sample Preparation
After collection of GCF, paper strips were removed and placed in reweighted Eppendorf tubes and kept at -20ºC until analysis. Before the HPLC analysis, GCF samples were thawed at room temperature. 15 μL from 100 μg mL-1 IS solution was added to the GCF sample. After adding acetonitrile as an extracting solvent up to volume of 200 μL, the GCF sample solutions were vortex mixed for 3 minutes. The liquid content of the tubes was transferred to glass autosampler vials. A 50-μL aliquot was injected into chromatographic system. The weight of the fluid was calculated as a difference between masses of the strips before GCF collection and after its application in the pocket. The obtained value, expressed as μg, was converted to volume in μL assuming the density of GCF was 1 mg mL-1 [11].
Statistical Analysis
The comparison of the values of the dental plaque and gingival inflammation at the beginning of the treatment and 15, 30 and 90 days after treatment were performed using Friedman ANOVA test (ANOVA Chi Square test). The difference in the values of dental plaque and gingival inflammation at the beginning and 90 days after treatment was tested using T-test for dependent samples (t) and Wilcoxon Matched pair test (Z), after testing for normal distribution. The difference in values of dental plaque and gingival inflammation between the two periodontal pockets one receiving SRP+ subgingival irrigation with 0.12 % CHX solution, and the other receiving SRP+ subgingival application of PerioChip was tested using Mann-Whitney U test (Z) and T-test for independent samples (t). The level of significance was set at p < 0>
Results
The differences in dental plaque values at baseline and 15, 30 and 90 days after the two treatment modalities, SRP followed by application of 0.12 % CHX solution and SRP followed by insertion of PerioChip, are shown in Table 1. For ANOVA Chi Sqr. = 90.00 and p <0 xss=removed xss=removed xss=removed>
Table 1: Differences between dental plaque index at baseline and 15, 30 and 90 days after treatment.
| Plaque index | SRP+0.12 % CHX Solution | SRP+ PerioChip | ||||||
| Average Rank | Sum of Ranks | Mean | SD | Average Rank | Sum of Ranks | Mean | SD | |
| Baseline | 4,00 | 120,00 | 1,62 | 0,30 | 3,97 | 119,00 | 1,44 | 0,28 |
| 15th day | 1,00 | 30,00 | 0,00 | 1,43 | 43,00 | 0,00 | ||
| 30th day | 2,00 | 60,00 | 0,29 | 0,13 | 1,93 | 58,00 | 0,06 | 0,08 |
| 90th day | 3,00 | 90,00 | 0,54 | 0,14 | 2,67 | 80,00 | 0,24 | 0,72 |
*SD-Standard Deviation
The average amount of dental plaque (Mean = 0,54) 90 days after SRP treatment followed by application of 0.12 % CHX solution for (t = 23,18 and p less than 0,001 (p = 0,000)) was significantly lower compared to the average value of dental plaque (mean = 1.62) at baseline. Similarly, the average amount of dental plaque (Mean = 0,24) 90 days after SRP + PerioChip treatment for Z = 4,17 and p less than 0,001 (p = 0,000) was significantly lower compared to the average value of dental plaque (Mean = 1,44) at baseline (Table 2).
Table 2: Differences between the dental plaque values at baseline and 90 days after treatment.
| Dental Plaque | SRP+0.12 % CHX Solution | SRP+ PerioChip | ||||||
| Baseline | Valid | T | Z | p-level | Valid | T | Z | p-level |
| 90th day | 30,00 | 23,18 | 29 | 0,000 | 30 | 30,00 | 4,17 | 0,000 |
The differences in gingival inflammation index at baseline, and 15, 30 and 90 days after both treatment modalities, SRP followed by subgingival irrigation with 0.12 % CHX solution and SRP followed by subgingival insertion of the Periochip, were statistically significant. Namely at all tested intervals, the gingival inflammation decreases. Results are shown in Table 3. For ANOVA Chi Sqr. = 83,38 and p less than 0,001 (p = 0,000) there is a significant difference between gingival inflammation after subgingival irrigation with 0.12 % CHX solution. 15 days (0,61 ± 0,22), 30 days (0,65 ± 0,21) and 90 days (0,72 ± 0,19) after the application of 0.12 % CHX solution the gingival inflammation is significantly lower compared to baseline. As for the differences between the values of gingival inflammation at baseline and 15, 30 and 90 days after treatment with SRP followed by subgingival insertion of the PerioChip yilded ANOVA Chi Sqr. = 85,20 and p less than 0,001 (p = 0,000) meaning that there is a significant difference between the analyzed values for gingival inflammation. Gingival inflammation varies in the range 0,13 ± 0,08 15 days after treatment, 30 days after treatment the variation is 0,02 ± 0,03, and 90 days after therapy is the variation is in the range 0,004 ± 0,01.
Table 3: Differences between gingival inflammation index at baseline and 15, 30 and 90 days after treatment.
| Gingival Inflammation Index | SRP+0.12 % CHX Solution | SRP+ PerioChip | ||||||
| Average Rank | Sum of Ranks | Mean | SD | Average Rank | Sum of Ranks | Mean | SD | |
| Baseline | 4,00 | 120,00 | 1,55 | 0,26 | 4,00 | 120,00 | 1,54 | 0,25 |
| 15th day | 1,17 | 35,00 | 0,61 | 0,22 | 2,90 | 87,000 | 0,13 | 0,08 |
| 30th day | 1,93 | 58,00 | 0,65 | 0,21 | 1,70 | 51,00 | 0,02 | 0,03 |
| 90th day | 2,90 | 87,00 | 0,72 | 0,19 | 1,40 | 42,00 | 0,004 | 0,01 |
*SD-Standard Deviation
The average value of gingival inflammation index (Mean=0,72) 90 days after treatment with SRP followed by subgingival irrigation of 0.12% CHX solution applied to Z=4,78 and p less than 0,001 (p=0,000) was significantly lower compared to the average of gingival inflammation (Mean=1.55) at the beginning of the treatment. The average value of gingival inflammation index (Mean = 0,004) 90 days after treatment with SRP followed by subgingival insertion of PerioChip for Z=4,78 and p less than 0,001 (p = 0,000) was significantly lower compared to the average of gingival inflammation index (Mean=1,54) at baseline. Results are presented in Table 4.
Table 4: Differences between the gingival inflammation index values at baseline and 90 days after treatment.
| Gingival Inflammation Index | SRP+0.12 % CHX Solution | SRP+ PerioChip | ||||||
| Baseline | Valid | T | Z | p-level | Valid | T | Z | p-level |
| 90th day | 30 | 0,00 | 4,78 | 0,000 | 30 | 0,00 | 4,78 | 0,000 |
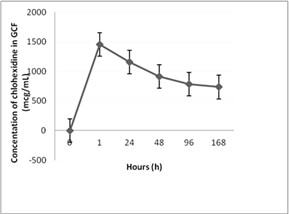
After optimization and validation of the HPLC method for determination of CHX in GCF samples, the method was applied in routine analysis in order to determine the concentration of CHX in GCF in periodontitis patients after the application of the PerioChip and after the subgingival irrigation with the 0.12 % CHX solution. It was found that the highest concentrations of CHX in GCF are achieved 1 hour after application of the PerioChip, while concentrations in the range of 1200.3-500.5 μg mL-1 are retained until day 7 post chip application. After two to four days CHX concentrations reach values up to 1200.3 μg mL-1, and after that interval, they gradually decline but remain above 125 μg mL-1. This concentration is minimal inhibitory concentration (MIC) by more than 99% of peri pathogenic microorganisms most frequently found in periodontal pockets. Results are expressed as the mean concentrations determined (n = 3) ± standard error and are shown graphically in Figure 1. However, after application of 0.12% chlorhexidine solution in the selected pockets, the CHX concentrations were below the limit of detection. This is probably due to the low concentration of drug in dosage form, and the type of dosage form of chlorhexidine (irrigation solution) which cannot sustain biologically significant concentrations of the drug in periodontal pockets for longer period of time.
Figure 1: Graphical presentation of the mean concentrations of CHX in GCF during the test interval.
Discussion
The results from our study show that SRP followed by subgingival irrigation of 0.12 % CHX solution results in decrease of dental plaque index during the test period. In fact, 15 days after treatment dental plaque is not registered. 30 and 90 days after treatment the dental plaque index varies in the interval 0,29 ± 0,13 and 0,54 ± 0,14, respectively. In both cases the values are lower compared to baseline. The application of SRP + PerioChip after 15, 30 and 90 days in rehab for ANOVA Chi Sqr. = 90.00 and p <0 xss=removed xss=removed xss=removed>
The decrease in dental plaque index, 90 days after the beginning of the both treatment modalities are as a result of the antibacterial action of CHX that complement the conventional, non-surgical treatment of the periodontal pockets. In the current study, greater decrease in plaque index can be seen at the sites treated with SRP followed by subgingival insertion of the PerioChip. This is consistent with the findings that CHX can be especially effective when used as a sustained delivery system applied locally, in the periodontal pockets [16]. In vitro studies on the release of CHX from the carrier have shown that 40% of the incorporated CHX is released within 24 hours, and the rest in the next 7-10 days. The concentration of 7-day period was 125 µg mL-1, in contrast to level of 1452.8 µg mL-1 for 4 hours and 783.6 µg mL-1 for 3 days. Studies have shown suppression of subgingival flora for a period of 11 weeks after treatment with Periochip. The results obtained are consistent with the findings of Killoy16. We believe that the findings of reduced dental plaque and positive effect on gingival inflammation due to sustained release of CHX in an extended period of time, which confirms the effect of MIC on the subgingival microflora. In fact, the sustained release delivery device slow-degrading continuous process and ensures the delivery of the drug in the periodontal pocket more than one day [17,18]. In our study the application of 0.12% chlorhexidine solution in the selected pockets resulted in CHX concentrations below the limit of detection, hence the mechanical elimination of microorganisms and local short-term effect of chlorhexidine solution resulted in limited improvement of clinical parameters compared to the application of the PerioChip.
As for gingival inflammation after 15 days (0,61 ± 0,22), 30 days (0,65 ± 0,21) and 90 days (0,72 ± 0,19) of applied SRP + subgingival irrigation with 0.12 % CHX solution, the average of gingival inflammation varies and is significantly lower than the values at the first examination, while the differences between the values of gingival inflammation in relation first review after 15, 30 and 90 days in rehab, the treatment with the PerioChip is proven that ANOVA Chi Sqr. = 85,20 and p <0 xss=removed>
Conclusion
In conclusion, the clinical data in the present study indicate that the application of the PerioChip adjunctive to SRP might be beneficial in improving clinical periodontal parameters. However, the application of local delivery systems which contain antimicrobial agents does not replace the need for thorough SRP, which remains the most important and the primary treatment modality. The local application of the PerioChip along with SRP represents a simple and non-invasive technique which can be used as a beneficial adjunctive treatment modality to enhance periodontal health or reduce the risk of periodontal disease reccurence.
References
- Matesanz P, Herrera D, Echverria A, et al. (2012). A Randomized Clinical Trial on The Clinical and Microbiological Efficacy of a Xanthan Gel with Chlorhexidine for Subgingival Use. Clin Oral Invest. 17:55-66.
Publisher | Google Scholor - Haffajee AD, Socranscy SS, Patel MR, et al. (2008). Microbial Complexes in Supragingival Plaque. J Oral Microbiol Immunol, 23:196-205.
Publisher | Google Scholor - Lindhe J. (2004). Clinical Periodontology and Implant Dentistry. Blackwell Munsgaard Publishing, 476:110-128.
Publisher | Google Scholor - Cosyn J, Win I, De Rouck T, et al. (2006). Clinical Benefits of Subgingival Chlorhexidine Varnish Application as An Adjunct to Same Day Full-Mouth Root Planning: A Pilot Study. J Periodontol. 77:1074-1079.
Publisher | Google Scholor - Jamilian A, Ghasemi M, Gholami D, et al. (2008). Clinical Effects of 2 % Chlorhexidine Gel on Patients Undergoing Orthodontic Treatment. Orthod Waves. 67:162-168.
Publisher | Google Scholor - Drisko CH. (2000). Nonsurgical Periodontal Therapy. Periodontol. 25:77-88.
Publisher | Google Scholor - Slots J. (2002). Selection of Antimicrobial Agents in Periodontal Therapy. J Periodont Res. 37:389-398.
Publisher | Google Scholor - Perrineti G, Paolantonio M, Cordella C, et al. (2004). Clinical and Microbiological Effects of Subgingival Administration of Two Active Gels on Persistent Pockets of Chronic Periodontitis Patients. J Clin Periodontol. 31:273-281.
Publisher | Google Scholor - Loe H. (1967). The Gingival Index, The Plaque Index and The Retention Systems. J Periodontol. 38:610-616.
Publisher | Google Scholor - Turesky S, Gilmore ND, Glickman I. (1970). Reduced Plaque Formation by The Chloromethyl Analogue of Vitamin C. J Periodontol. 41:41-43.
Publisher | Google Scholor - Koss M, Castro C, Salum K, et al. (2009). Enzymatic Profile of Gingival Crevicular Fluid in Association with Periodontal Status. Lab Med. 40:277-280.
Publisher | Google Scholor - Stabholz A, Sela MN, Friedman, et al. (1986). Clinical and Microbiological Effects of a Sustained Release Chlorhexidine in Periodontal Pockets. J Clin Periodontol. 13:783-788.
Publisher | Google Scholor - Soskolne WA, Heasman PA, Stabholz A, et al. (1997). Sustained Local Delivery of Chlorhexidine in The Treatment of Periodontitis. J Periodontol. 68:32-38.
Publisher | Google Scholor - Jeffcoat MK, Bray KS, Ciancio SG, et al. (1998). Adjunctive Use of a Subgingival Controlled Release Chlorhexidine Chip Reduces Probing Depth and Improves Attachment Level Compared to Scaling and Root Planning Alone. J Periodontol. 69:989-997.
Publisher | Google Scholor - Heasman PA, Heasman L, Stacey F, et al. (2001). Local Delivery of Chlorhexidine Gluconate (Periochip) In Periodontal Maintainence Patients. J Clin Periodontol. 28:90-95.
Publisher | Google Scholor - Killoy WJ. (1998). The Use of Locally Delivered Chlorhexidine in The Treatment of Periodontitis. Clinical Results. J Clin Periodontol. 25:953-958.
Publisher | Google Scholor - Slots J, Rams TE. (1990). Antibiotics In Periodontal Therapy: Advantages and Disadvantages. J Clin Periodontol. 17:479-493.
Publisher | Google Scholor - Ciancio SG. (1999). Local Delivery of Chlorhexidine. Comped Contin Educ Dent. 20:427-432.
Publisher | Google Scholor - Grover V, Kapoor A, Malhotra R, et al. (2011). To Assess the Effectiveness of a Chlorhexidine Chip in The Treatment of Chronic Periodontitis: A Clinical and Radiographic Study. J Indian Soc Periodontol. 2011 15:139-146.
Publisher | Google Scholor - Azmak N, Atilla G, Luoto H, et al. (2002). The Effect of Subgingival Controlled Release Delivery of Chlorhexidine Chip on Clinical Parameters and Matrix Metalloproteinase-8 Levels in Gingival Crevicular Fluid. J Periodontol. 73:608-615.
Publisher | Google Scholor - De Graaff J, van Winkelhoff AJ, Goen RJ. (1989). The role of Actinobacillus actinomycetemcomitans in periodontal disease. Infection. 17:269-271.
Publisher | Google Scholor - Kornman KS. (1993). Controlled-Release Local Delivery Antimicrobials in Periodontics: Prospects for The Future. J Periodontol. 64:782-791.
Publisher | Google Scholor