Research Article
ERN1-Dependent Sensitivity of CEBPB and other Gene Expressions to Glutamine Deprivation in U87MG Glioblastoma Cells
- Oleksandr H. Minchenko 1*
- Yulia M. Viletska 1
- Oksana O. Ratushna 1
- Serhiy V. Danilovskyi 1
- Halyna E. Kozynkevych 2
- Borys H. Bezrodnyi 2
- Dmytro O. Minchenko 1,2
1Palladin Institute of Biochemistry of the National Academy of Sciences of Ukraine, Kyiv, Ukraine.
2National Bohomolets Medical University, Kyiv, Ukraine.
*Corresponding Author: Oleksandr H. Minchenko, Palladin Institute of Biochemistry of the National Academy of Sciences of Ukraine, Kyiv, Ukraine.
Citation: Minchenko O H, Viletska Y M, Ratushna O O, Danilovskyi S V, Kozynkevych H E, et, al. (2024). ERN1-Dependent Sensitivity of CEBPB and other Gene Expressions to Glutamine Deprivation in U87MG Glioblastoma Cells. Journal of Endocrinology and Diabetes Research, BioRes Scientia Publishers. 2(3):1-10. DOI: 10.59657/2996-3095.brs.24.018
Copyright: © 2024 Oleksandr H. Minchenko, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: November 11, 2024 | Accepted: December 07, 2024 | Published: December 19, 2024
Abstract
We studied the impact of glutamine deprivation on the expression of CEBPB and several other genes such as CEBPG, PTEN, CKS2, and CCNH in U87MG glioblastoma cells in response to ERN1 knockdown for evaluation of the significance of ERN1 signaling in the control of glioblastoma cell sensitivity to nutrient supply. It was shown that the expression level of CEBPB, CEBPG, and PTEN genes was up-regulated in control glioblastoma cells treated by glutamine deprivation, but ERN1 knockdown enhanced the sensitivity of all these genes to this experimental condition. At the same time, the expression of CKS2 and CCNH genes is decreased in control glioblastoma cells by glutamine deprivation, but inhibition of ERN1 increases the impact of this deprivation condition on CKS2 gene and removes the sensitivity of CCNH gene. These results demonstrate that ERN1 controls the sensitivity of all studied genes to glutamine deprivation in glioblastoma cell in gene-specific manner.
Keywords: ERN1 knockdown; CEBPB; PTEN; mRNA expression; Glutamine deprivation; U87MG glioblastoma cells
Introduction
Glutamine is the most abundant amino acid in tissues and represents an essential substrate for tumor cell metabolism as a substrate for glycolysis. Reliance on glutamine has been considered a hallmark of metabolism in many cancer cells, but the requirements for glutamine in cancer are heterogeneous (Colombo et al. 2011; Cluntun et al. 2017; Kodama et al. 2020; Bhowmick et al. 2023; Li et al. 2023). Glutamine is important for the glioblastoma development and a more aggressive behavior (Daye and Wellen, 2012; Guo et al. 2016; Zhao et al. 2017; Yoo et al. 2020; Minchenko et al. 2021a). There is mounting evidence that there is a link between glutamine metabolism and the proliferation of cancer cells, demonstrating that glutamine metabolism is a vital mechanism for all cellular processes, including the development of cancer (Buono and Longo 2018; Fasoulakis et al. 2023; Jin et al. 2023). Moreover, uptake of glutamine and subsequent glutaminolysis is significant for activation of the mTORC1 pathway, which regulates cell proliferation in triple-negative basal-like breast cancer (van Geldermalsen et al. 2016). It is interesting to note that glutamine deprivation as well as endoplasmic reticulum stress are very important and complementary factors for tumor growth and that ERN1/IRE1 (endoplasmic reticulum to nucleus signaling 1/inositol requiring enzyme 1) mediated stress signaling can significantly modify the effects of glutamine deprivation on gene expressions (Drogat et al. 2007; Iurlaro et al. 2017; Minchenko et al. 2018; Jiang et al. 2019; Teramoto et al. 2019; Krasnytska et al. 2022; Jin et al. 2023). Furthermore, ERN1 knockdown also modifies the impact of glucose deprivation on the expression of numerous factors related to insulin and glucocorticoid receptors (Minchenko et al. 2013, 2020, 2021a; Riabovol et al. 2019; Tsymbal et al. 2020).
It has also been shown that ERN1/XBP1 pathway is essential for the glutamine response and protection of β cells (Hassler et al. 2015). Bioinformatic profiling identifies a glutamine-related risk signature for the malignancy of glioblastoma and the survival of patients (Zhao et al. 2017). However, the detailed molecular mechanisms of the interaction of glutamine deprivation with ERN1 mediated stress signaling pathway are complex and warrant further study. Furthermore, there are data indicating that glutamine is an important factor for cancer cells chemoresistance (Awale et al. 2006). Therefore, arctigenin inhibits numerous cancer cells growth and induces tumor cell death under glutamine deprivation possibly by suppressing the endoplasmic reticulum stress (Awale et al. 2006; Kim et al. 2010; Gu et al. 2012; He et al. 2018). This antitumor agent remove the tolerance of cancer cells to deprivation of glutamine, but mechanism of suppression of chemoresistance upon this condition is unknown yet. Moreover, deprivation of glutamine in Balb 3T3 cell culture reveals its potential for treating experimental and human cancers (Rubin et al. 2019). Interestingly, low-glucose also protected primary glial cells but not six different glioblastoma and neuroblastoma cancer cell lines against the chemotherapy (Raffaghello et al. 2008). Moreover, the uptake of glutamine and subsequent glutaminolysis is critical for activating signaling pathways, controlling tumor growth. This process is largely controlled by SLC1A5 (solute carrier family 1 member 5), which is responsible for the transport of glutamine, serine and some other amino acids and involved in the control of tumor growth (van Geldermalsen et al. 2016; Wang et al. 2019; Minchenko et al. 2023).
Transcription factors CEBPB and CEBPG (CCAAT enhancer binding proteins) play an important role in the regulation of genes involved in numerous metabolic processes including gluconeogenesis and immune response (Huber et al. 2012; Li et al. 2024; Ma et al. 2024; Zhang et al. 2024a). Phosphatase and tensin homolog (PTEN) is a multi-functional tumor suppressor that is very commonly lost in human cancer, including glioblastoma (Wang et al. 2018). It regulates insulin signaling and glucose metabolism in adipose tissue (Molina-Ayala et al. 2022). CDC28 protein kinase regulatory subunit 2 (CKS2) binds to the catalytic subunit of the cyclin dependent kinases and is essential for malignant phenotypes and epithelial-mesenchymal transition-like process in glioblastoma by activating TGFbeta/SMAD signaling (Feng et al. 2023). Cyclin H (CCNH) functions as regulator of CDK kinases, involved in cell cycle control, participates in transcriptional regulation, and tumor growth (Mao et al. 2021; Liu et al. 2022).
Malignant tumors use endoplasmic reticulum stress response and its signaling pathways to adapt and to enhance tumor cells proliferation under stressful environmental conditions including low glutamine (Bravo et al. 2013; Huber et al. 2013; Almanza et al. 2019). It is well known that activation of ERN1 branch of the endoplasmic reticulum stress response is tightly linked to apoptosis and to cell death, and inhibition of its function has been demonstrated to result in significant anti-proliferative effect in glioblastoma growth (Auf et al. 2010, 2013; Logue et al. 2018; Hetz et al. 2019; Minchenko et al. 2024a, b).
The aim of this study was to examine the expression of important regulatory genes in response to glutamine deprivation in control and ERN1 knockdown glioblastoma cells for evaluation of possible significance of this deprivation condition in the control of cell proliferation mediated ERN1 signaling.
Materials and Methods
Cell lines and culture conditions
The glioblastoma cell line U87MG was obtained from ATCC (USA) and grown in high glutamine (4.5 g/l) Dulbecco’s modified Eagle’s minimum essential medium (Gibco, Invitrogen, Carlsbad, CA, USA) supplemented with glutamine (2 mM), 10 Percentage fetal bovine serum (Equitech-Bio, Inc., USA), penicillin (100 units/ml; Gibco) and streptomycin (0.1 mg/ml; Gibco) at 37oC in incubator with 5Percentage CO2. In this work we used two sublines of these cells described previously (Auf et al. 2013). One subline was obtained by selection of stable transfected clones with overexpression of vector pcDNA3.1, which was used for creation of dnERN1. This untreated subline of glioblastoma cells (control glioblastoma cells) was used as control 1 in the study of the effect of glutamine deprivation on the level of gene expressions. Second subline was obtained by selection of stable transfected clones with overexpression of ERN1 dominant/negative construct (dnERN1), having suppression of both the protein kinase and endoribonuclease activities of this signaling enzyme (Auf et al. 2013). It has been shown that cells with dnERN1 have a lower proliferation rate, do not express spliced XBP1, a key transcription factor in ERN1 signaling, and have not the phosphorylated isoform of ERN1 after induction of endoplasmic reticulum stress by tunicamycin (Auf et al. 2013). Both used in this study sublines of glioblastoma cells are grown in the presence of geneticin (G418) while these cells carrying empty vector pcDNA3.1 or dnERN1 construct. Glutamine deprivation condition were created by changing the complete DMEM medium into culture plates on DMEM medium without glutamine and plates were exposed to this condition for 16h.
RNA isolation. Total RNA was extracted from glioblastoma cells using the Trizol reagent according to manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA) as described previously (Auf et al. 2013).
Reverse transcription and quantitative PCR analysis. The expression levels of CEBPB, CEBPG, PTEN, CKS2, and CCNH mRNAs as well as ACTB mRNA were measured in control U87MG glioblastoma cells and cells with a deficiency of ERN1, introduced by dnERN1, by quantitative polymerase chain reaction using SYBRGreen Mix (ABgene, Thermo Fisher Scientific, Epsom, Surrey, UK) and “QuantStudio 5 Real-Time PCR System” (Applied Biosystems, USA). Thermo Scientific Verso cDNA Synthesis Kit (Germany) was used for reverse transcription as described previously (Minchenko et al. 2019). Polymerase chain reaction was performed in triplicate. The expression of beta-actin mRNA was used as control of analyzed RNA quantity. The pair of primers specific for each studied gene was received from Sigma-Aldrich (St. Louis, MO, U.S.A.) and used for quantitative polymerase chain reaction (Table 1).
Table 1: Characteristics of the primers used for quantitative real-time polymerase chain reaction.
| Gene symbol | Gene name | Primer’s sequence | Nucleotide number in sequence | GeneBank accession number |
| CEBPB | CCAAT enhancer binding protein beta | F:5’- caagaagaccgtggacaagc | 993-1012 | NM_005194.4 |
| R:5’- agctgctccaccttcttctg | 1163-1144 | |||
| CEBPG | CCAAT enhancer binding protein gamma | F:5’- aagcacaagacacactgcag | 540-559 | NM_001806.4 |
| R:5’- ctactgtcctgcattgtcgc | 736-717 | |||
| PTEN | Phosphatase and tensin homolog | F:5’- actattcccagtcagaggcg | 1344-1363 | NM_000314.8 |
| R:5’- gaacttgtcttcccgtcgtg | 1559-1540 | |||
| CKS2 | CDC28 protein kinase regulatory subunit 2 | F:5’- gtttcattttctgcagcgcg | 71-90 | NM_001827.3 |
| R:5’- ccaagtctcctccactcctc | 238-219 | |||
| CCNH | Cyclin H | F:5’- gttcggtgtttaagccagca | 339–358 | NM_001239.4 |
| R:5’- tgccttctcctgtccaagag | 553–534 | |||
| ACTB | beta-actin | F:5’- catccgcaaagacctgtacg | 948–967 | NM_001101.5 |
| R:5’- cctgcttgctgatccacatc | 1165–1146 |
Quantitative PCR analysis was performed using a special computer program “Differential expression calculator” and statistical analysis using Excel program and OriginPro 7.5 software as described previously (Rudnytska et al. 2021). Comparison of two means was performed by the use of two-tailed Student’s t-test. p<0> gene expressions were normalized to the expression of beta-actin mRNA and represent as percent of control (100%). All values are expressed as mean ± SEM from triplicate measurements performed in 4 independent experiments. The amplified DNA fragments were also analyzed on a 3% agarose gel and that visualized by SYBR* Safe DNA Gel Stain (Life Technologies, Carlsbad, CA, USA).
Results
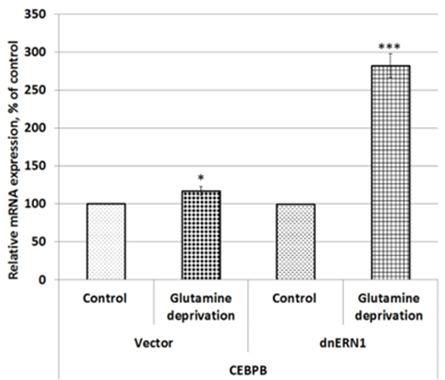
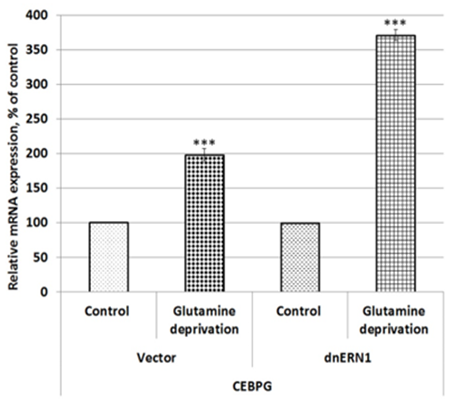
To investigate a possible role of endoplasmic reticulum stress response in the control of CEBPB, CEBPG, PTEN, CKS2, and CCNH gene expressions in U87MG glioblastoma cells under low glutamine condition we studied the impact of glutamine deprivation on the expression of these genes in control glioblastoma cells (transfected by empty vector) and cells with ERN1 knockdown. As shown in Figure 1, the expression of CCAAT enhancer binding protein beta (CEBPB) mRNA is increased in control glioblastoma cells after exposure under condition of glutamine deprivation (+17 %) in comparison with the cells growing in complete DMEM medium. Furthermore, inhibition of ERN1 signaling enzyme function by dnERN1 is significantly enhanced the sensitivity of CEBPB gene expression to this experimental condition. Thus, the level of this mRNA expression is increased more strongly (+182 %) in cells without the enzymatic activities of ERN1 signaling protein (Figure 1). Next, we investigated the effect of glutamine deprivation on the expression of gene encoding CCAAT enhancer binding protein gamma (CEBPG) in relation to inhibition of ERN1 function. As shown in Figure 2, glutamine deprivation condition results in significant up-regulation of this homeobox gene [removed]+98 %) in comparison with control glioblastoma cells, transfected by empty vector. At the same time, ERN1 knockdown strongly increases the sensitivity of this gene expression to glutamine deprivation conditions (+271 %; Figure 2).
Figure 1: Effect of glutamine deprivation on the expression level of CCAAT enhancer binding protein beta (CEBPB) mRNA in control U87 glioblastoma cells (Vector) and cells with a blockade of the ERN1 (dnERN1) measured by qPCR. Values of this mRNA expression were normalized to beta-actin mRNA level and represented as percent for control 1(100%); n=4; *- p less than 0.05; p less than 0.001.
Figure 2: Effect of glutamine deprivation on the expression level of CCAAT enhancer binding protein gamma (CEBPG) mRNA in control U87 glioblastoma cells (Vector) and cells with a blockade of the ERN1 (dnERN1) measured by qPCR. Values of this mRNA expression were normalized to beta-actin mRNA level and represented as percent for control 1 (100%); n=4; ***- p less than 0.001.
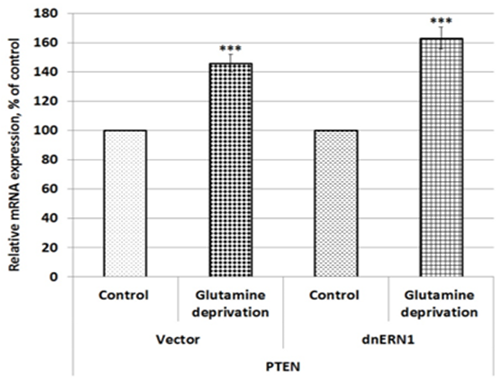
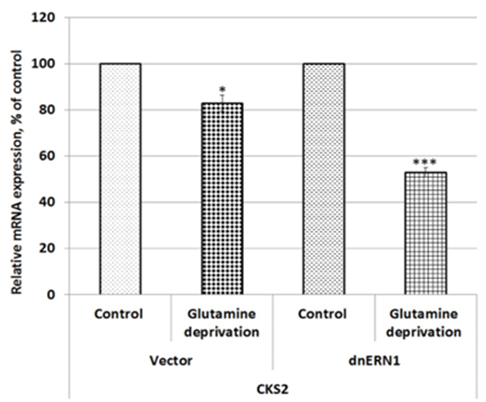
We also investigated the effect of glutamine deprivation condition on the expression of PTEN gene (phosphatase and tensin homolog) in U87MG glioblastoma cells in relation to ERN1 knockdown. As shown in Figure 3, the expression of PTEN mRNA is also up-regulated in control glioblastoma cells treated by glutamine deprivation (+46 %) and inhibition of enzymatic activities of ERN1 signaling protein increased the sensitivity of this mRNA expression to glutamine deprivation in comparison with dnERN1 cells growing with glutamine. At the same time, the expression level of CKS2 gene encoding CDC28 protein kinase regulatory subunit 2 is down-regulated (-17 %) under glutamine deprivation condition in control glioblastoma cells in comparison with cells growing in regular medium (Figure 4). Furthermore, glutamine deprivation of ERN1 knockdown glioblastoma cells introduces stronger suppression of this gene [removed]-47 %) as compared to ERN1 knockdown cells growing with glutamine (Figure 4).
Figure 3: Effect of glutamine deprivation on the expression level of phosphatase and tensin homolog (PTEN) mRNA in control U87 glioblastoma cells (Vector) and cells with a blockade of the ERN1 (dnERN1) measured by qPCR. Values of this mRNA expression were normalized to beta-actin mRNA level and represented as percent for control 1 (100%); n=4; ***p less than 0.001.
Figure 4: Effect of glutamine deprivation on the expression level of CDC28 protein kinase regulatory subunit 2 (CKS2) mRNA in control U87 glioblastoma cells (Vector) and cells with a blockade of the ERN1 (dnERN1) measured by qPCR. Values of this mRNA expression were normalized to beta-actin mRNA level and represented as percent for control 1 (100%); n=4; *- p less than 0.05.
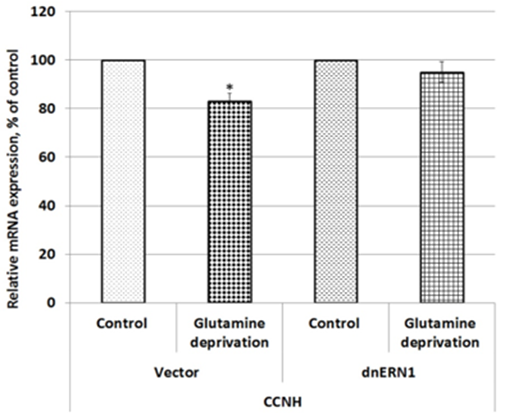
We have also studied the effect of glutamine deprivation on the expression of cyclin H gene in control and ERN1 knockdown glioblastoma cells. As shown in Figure 5, exposure of glioblastoma cells under glutamine deprivation condition leads to down-regulation of CCNH mRNA expression (-17 %) in comparison with control cells growing under condition with glutamine. Furthermore, inhibition of ERN1 completely removed the sensitivity of this gene expression to glutamine deprivation.
Figure 5: Effect of glutamine deprivation on the expression level of Cyclin H (CCNH) mRNA in control U87 glioblastoma cells (Vector) and cells with a blockade of the ERN1 (dnERN1) measured by qPCR. Values of this mRNA expression were normalized to beta-actin mRNA level and represented as percent for control 1 (100%); n=4; *- p less than 0.05.
Thus, glutamine deprivation condition affects the expression of CEBPB, CEBPG, PTEN, CKS2, and CCNH genes in gene-specific manner and the impact of glutamine deprivation condition on most gene expressions depends on ERN1 signaling.
Discussion
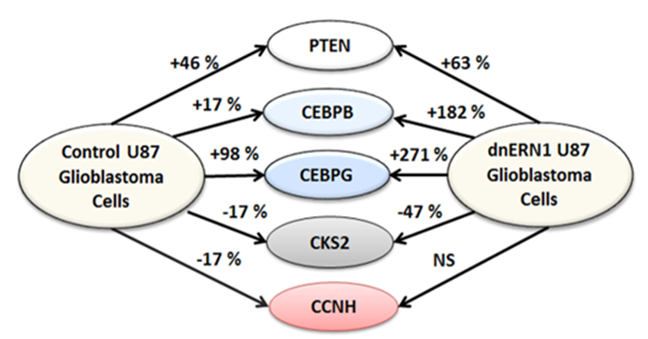
In this work, we studied the effect of glutamine deprivation on the expression of genes encoding important regulatory proteins in U87MG glioblastoma cells in relation to inhibition of ERN1, the major signaling pathway of the unfolded protein response. For this aim we used control glioblastoma cells, transfected by empty vector pcDNA3.1 and cells with full ERN1 deficiency introduced by dnERN1. This is important for the evaluation of possible significance of in ERN1 dependent control of glioblastoma cell proliferation because endoplasmic reticulum stress signaling mediated by ERN1 is involved in numerous metabolic pathways and ERN1 knockdown has clear anti-tumor effects (Auf et al. 2010, 2013; Bravo et al. 2013; Logue et al. 2018; Almanza et al. 2019; Minchenko et al. 2021b). Furthermore, there are data that glutamine deprivation can enhance the sensitivity of cancer cells to anti-cancer drugs, particularly arctigenin, which inhibits the growth of various cancer cells and induces tumor cell death under glutamine deprivation condition possibly by blocking the unfolded protein response and inhibiting cellular energy metabolism (Awale et al. 2006; Kim et al. 2010; Gu et al. 2012; He et al. 2018). Results of our study clarify possible mechanisms of glutamine deprivation on the proliferation/surviving of ERN1 knockdown glioblastoma cells through specific changes in the expression of genes encoding important regulatory proteins. Results of this investigation are summarized in Figure 6, which clearly demonstrated the differential effect of glutamine deprivation on the expression of CEBPB, CEBPG, PTEN, CKS2, and CCNH genes and ERN1-dependent character of changes in the expression profile of all studied genes in glioblastoma cells under glutamine deprivation in gene-specific manner. Therefore, eliminating CCNH gene expression sensitivity to glutamine deprivation in ERN1 knockdown glioblastoma cells may contribute to decreased proliferation potential of these cells (Mao et al. 2021; Minchenko et al. 2024a). More strong suppression of CKS2gene expression in glioblastoma cells with knockdown of ERN1 can also introduce decreased cell proliferation of these cells (Feng et al. 2023; Zhang et al. 2024b).
Figure 6: Schematic demonstration of changes in the expression profile of PTEN, CEBPB, CEBPG, CKS2, and CCNH genes in the control and ERN1 knockdown (dnERN1) glioblastoma cells under glutamine deprivation; NS-no significant changes.
Furthermore, we showed that the expression of multi-functional tumor suppressor PTEN (phosphatase and tensin homolog) is also increased in glioblastoma cells with inhibited enzymatic activities of ERN1 as compared to control cells (Figure 6). It agrees well with data Wang et al. (2018) that PTEN down-regulation promoted the growth of cancer cells. As shown in Figure 6, glutamine deprivation introduces up-regulation of CEBPB and CEBPG gene expressions in control glioblastoma cells, but inhibition of ERN1 signaling leads to much stronger induction of these transcription regulators, which exerted multiple biological functions (Huber et al. 2012; Li et al. 2024; Ma et al. 2024; Zhang et al. 2024a). A strong increase in CEBPB gene expression may be associated with the regulation of genes involved in glycolysis and immune responses, among other processes (Li et al. 2018; Zhang et al. 2024a). Previously was shown that the expression of SLC1A5, which controls glutamine transport, is of great importance, as it showed pronounced dependence on the inhibition of ERN1 (Minchenko et al. 2023). It is possible that ERN1 controls glutamine uptake and metabolism through modulation of numerous gene expressions (Hassler et al. 2015; van Geldermalsen et al. 2016; Jiang et al. 2019; Wang et al. 2019).
This study provides unique insights into the molecular mechanisms regulating the expression of genes encoding important regulatory proteins in glioblastoma cells in response to glutamine deprivation and their correlation with inhibition of ERN1 activity and reduced cell proliferation in cells harboring dnERN1, attesting to the fact that endoplasmic reticulum stress as well as glutamine is a necessary component of malignant tumor growth and cell survival. Furthermore, our results validate tight interaction of endoplasmic reticulum stress signaling pathway ERN1 with glutamine deprivation in the regulation of the expression of genes encoding regulatory proteins, but the detailed molecular mechanisms of this regulation have not been yet clearly defined and requires further investigation.
Declarations
Acknowledgement
This work was funded by the State Budget Program "Support for the Development of Priority Areas of Scientific Research" (Code: 6541030).
Competing interest
All authors declare that they reviewed the manuscript and have no competing interest.
References
- Almanza A, Carlesso A, Chintha C, Creedican S, Doultsinos D, Leuzzi B, Luís A, McCarthy N, Montibeller L, More S, Papaioannou A, Püschel F, Sassano ML, Skoko J, Agostinis P, de Belleroche J, Eriksson LA, Fulda S, Gorman AM, Healy S, Kozlov A, Muñoz-Pinedo C, Rehm M, Chevet E, Samali A. (2019). Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J, 286:241-278.
Publisher | Google Scholor - Auf G, Jabouille A, Delugin M, Guérit S, Pineau R, North S, Platonova N, Maitre M, Favereaux A, Vajkoczy P, Seno M, Bikfalvi A, Minchenko D, Minchenko O, Moenner M. (2013). High epiregulin expression in human U87 glioma cells relies on IRE1alpha and promotes autocrine growth through EGF receptor. BMC Cancer, 13:597.
Publisher | Google Scholor - Auf G, Jabouille A, Guérit S, Pineau R, Delugin M, Bouchecareilh M, Favereaux A, Maitre M, Gaiser T, von Deimling A, Czabanka M, Vajkoczy P, Chevet E, Bikfalvi A, Moenner M. (2010). A shift from an angiogenic to invasive phenotype induced in malignant glioma by inhibition of the unfolded protein response sensor IRE1. Proc Natl Acad Sci U S A, 107:15553-15558.
Publisher | Google Scholor - Awale S, Lu J, Kalauni SK, Kurashima Y, Tezuka Y, Kadota S, Esumi H. (2006). Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation. Cancer Res, 66:1751-1757.
Publisher | Google Scholor - Bhowmick N, Posadas E, Ellis L, Freedland SJ, Vizio DD, Freeman MR, Theodorescu D, Figlin R, Gong J. (2023). Targeting Glutamine Metabolism in Prostate Cancer. Front Biosci (Elite Ed),15:2.
Publisher | Google Scholor - Bravo R1, Parra V, Gatica D, Rodriguez AE, Torrealba N, Paredes F, Wang ZV, Zorzano A, Hill JA, Jaimovich E, Quest AF, Lavandero S. (2013). Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol, 301:215-290.
Publisher | Google Scholor - Buono R, Longo VD. (2018). Starvation, Stress Resistance, and Cancer. Trends Endocrinol Metab, 29:271-280.
Publisher | Google Scholor - Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. (2017). Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer, 3:169-180.
Publisher | Google Scholor - Colombo SL, Palacios-Callender M, Frakich N, Carcamo S, Kovacs I, Tudzarova S, Moncada S. (2011). Molecular basis for the differential use of glucose and glutamine in cell proliferation as revealed by synchronized HeLa cells. Proc Natl Acad Sci USA, 108:21069-21074.
Publisher | Google Scholor - Daye D, Wellen KE. (2012). Metabolic reprogramming in cancer: unraveling the role of glutamine in tumorigenesis. Semin Cell Dev Biol, 23:362-369.
Publisher | Google Scholor - Drogat B, Bouchecareilh M, North S, Petibois C, Déléris G, Chevet E, Bikfalvi A, Moenner M. (2007). Acute L-glutamine deprivation compromises VEGF-a upregulation in A549/8 human carcinoma cells. J Cell Physiol, 212:463-472.
Publisher | Google Scholor - Fasoulakis Z, Koutras A, Ntounis T, Prokopakis I, Perros P, Chionis A, Sapantzoglou I, Katrachouras A, Konis K, Samara AA, Valsamaki A, Palios VC, Symeonidis P, Nikolettos K, Pagkalos A, Sotiriou S, Theodora M, Antsaklis P, Daskalakis G, Kontomanolis EN. ( 2023). Ovarian Cancer and Glutamine Metabolism. Int J Mol Sci, 24:5041.
Publisher | Google Scholor - Feng F, Zhao Z, Cai X, Heng X, Ma X. (2023). Cyclin-dependent kinase subunit2 (CKS2) promotes malignant phenotypes and epithelial-mesenchymal transition-like process in glioma by activating TGFbeta/SMAD signaling. Cancer Med, 12:5889-5907.
Publisher | Google Scholor - Gatenby RA, Gillies RJ. (2007). Glycolysis in cancer: A potential target for therapy. Int J Biochem Cell Biol, 39:1358-1366.
Publisher | Google Scholor - Gu Y, Qi C, Sun X, Ma X, Zhang H, Hu L, Yuan J, Yu Q. (2012). Arctigenin Preferentially Induces Tumor Cell Death Under Glucose Deprivation by Inhibiting Cellular Energy Metabolism. Biochem Pharmacol, 84:468-476.
Publisher | Google Scholor - Guo H, Nan Y, Zhen Y, Zhang Y, Guo L, Yu K, Huang Q, Zhong Y. (2016). miRNA-451 inhibits glioma cell proliferation and invasion by downregulating glutamine transporter 1. Tumour Biol, 37:13751-13761.
Publisher | Google Scholor - Hassler JR, Scheuner DL, Wang S, Han J, Kodali VK, Li P, Nguyen J, George JS, Davis C, Wu SP, Bai Y, Sartor M, Cavalcoli J, Malhi H, Baudouin G, Zhang Y, Yates Iii JR, Itkin-Ansari P, Volkmann N, Kaufman RJ. (2015). The IRE1α/XBP1s pathway is essential for the glutamine response and protection of β cells. PLoS Biol, 13:e1002277.
Publisher | Google Scholor - He Y, Fan Q, Cai T, Huang W, Xie X, Wen Y, Shi Z. (2018). Molecular Mechanisms of the Action of Arctigenin in Cancer. Biomed Pharmacother, 108:403-407.
Publisher | Google Scholor - Hensley CT, Wasti AT, DeBerardinis RJ. (2013). Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest, 123:3678-3684.
Publisher | Google Scholor - Hetz C, Axten JM, Patterson JB. (2019). Pharmacological targeting of the unfolded protein response for disease intervention. Nat Chem Biol, 15:764-775.
Publisher | Google Scholor - Huber R, Pietsch D, Panterodt T, Brand K. (2012). Regulation of C/EBPbeta and resulting functions in cells of the monocytic lineage. Cell Signal, 24:1287-1296.
Publisher | Google Scholor - Huber AL, Lebeau J, Guillaumot P, Petrilli V, Malek M, Chilloux J, Fauvet F, Payen L, Kfoury A, Renno T, Chevet E, Manie SN. (2013). p58(IPK)-mediated attenuation of the proapoptotic PERK-CHOP pathway allows malignant progression under low glutamine. Mol Cell, 49:1049-1059.
Publisher | Google Scholor - Iurlaro R, Puschel F, Leon-Annicchiarico CL, O'Connor H, Martin SJ, Palou-Gramon D, Lucendo E, Munoz-Pinedo C. (2017). Glutamine deprivation induces ATF4-mediated apoptosis through TRAIL death receptors. Mol Cell Biol, 37:e00479-e00516.
Publisher | Google Scholor - Jiang J, Srivastava S, Zhang J. (2019). Starve Cancer Cells of Glutamine: Break the Spell or Make a Hungry Monster?. Cancers (Basel), 11:804.
Publisher | Google Scholor - Jin J, Byun JK, Choi YK, Park KG. (2023). Targeting glutamine metabolism as a therapeutic strategy for cancer. Exp Mol Med, 55:706-715.
Publisher | Google Scholor - Kim JY, Hwang JH, Cha MR, Yoon MY, Son ES, Tomida A, Ko B, Song SW, Shinya K, Hwang YI, Park HR. (2010). Arctigenin Blocks the Unfolded Protein Response and Shows Therapeutic Antitumor Activity. J Cell Physiol, 224:33-40.
Publisher | Google Scholor - Kodama M, Oshikawa K, Shimizu H, Yoshioka S, Takahashi M, Izumi Y, Bamba T, Tateishi C, Tomonaga T, Matsumoto M, Nakayama KI. (2020). A shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer. Nat Commun, 11:1320.
Publisher | Google Scholor - Krasnytska DA, Khita OO, Tsymbal DO, Luzina OY, Cherednychenko AA, Kozynkevych HE, Bezrodny BH, Minchenko DO. (2022). The impact of glutamine deprivation on the expression of MEIS3, SPAG4, LHX1, LHX2, and LHX6 genes in ERN1 knockdown U87 glioma cells. Endocr Reg, 56:38-47.
Publisher | Google Scholor - Li W, Tanikawa T, Kryczek I, Xia H, Li G, Wu K, Wei S, Zhao L, Vatan L, Wen B, Shu P, Sun D, Kleer C, Wicha M, Sabel M, Tao K, Wang G, Zou W. (2018). Aerobic Glycolysis Controls Myeloid-Derived Suppressor Cells and Tumor Immunity via a Specific CEBPB Isoform in Triple-Negative Breast Cancer. Cell Metab, 28:87-103.e6.
Publisher | Google Scholor - Li S, Zeng H, Fan J, Wang F, Xu C, Li Y, Tu J, Nephew KP, Long X. (2023). Glutamine metabolism in breast cancer and possible therapeutic targets. Biochem Pharmacol, 210:115464.
Publisher | Google Scholor - Li M, Ni QY, Yu SY. (2024). Integration of single-cell transcriptomics and epigenetic analysis reveals enhancer-controlled TIMP1 as a regulator of ferroptosis in colorectal cancer. Genes Genomics, 46:121-133.
Publisher | Google Scholor - Liu NQ, Cao WH, Wang X, Chen J, Nie J. (2022). Cyclin genes as potential novel prognostic biomarkers and therapeutic targets in breast cancer. Oncol Lett, 24:374.
Publisher | Google Scholor - Logue SE, McGrath EP, Cleary P, Greene S, Mnich K, Almanza A, Chevet E, Dwyer RM, Oommen A, Legembre P, Godey F, Madden EC, Leuzzi B, Obacz J, Zeng Q, Patterson JB, Jäger R, Gorman AM, Samali A. (2018). Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat Commun, 9:3267.
Publisher | Google Scholor - Ma Y, Chen Y, Zhan L, Dong Q, Wang Y, Li X, He L, Zhang J. (2024). CEBPB-mediated upregulation of SERPINA1 promotes colorectal cancer progression by enhancing STAT3 signaling. Cell Death Discov, 10:219.
Publisher | Google Scholor - Mao L, Ling X, Chen J. (2021). Cyclin H Regulates Lung Cancer Progression as a Carcinoma Inducer. Comput Math Methods Med, 6646077.
Publisher | Google Scholor - Minchenko DO, Kharkova AP, Hubenia OV, Minchenko OH. (2013). Insulin receptor, IRS1, IRS2, INSIG1, INSIG2, RRAD, and BAIAP2 gene expressions in glioma U87 cells with ERN1 loss of function: effect of hypoxia and glutamine or glucose deprivation. Endocr Reg, 47:15-26.
Publisher | Google Scholor - Minchenko OH, Kharkova AP, Hnatiuk OS, Luzina OY, Kryvdiuk IV, Kuznetsova AY. (2018). ERN1 modifies effect of glutamine deprivation on tumor growth related factors expression in U87 glioma cells. Ukr Biochem J, 90(3):49-61.
Publisher | Google Scholor - Minchenko DO, Khita OO, Tsymbal DO, Danilovskyi SV, Rudnytska OV, Halkin OV, Kryvdiuk IV, Smeshkova MV, Yakymchuk MM, Bezrodnyi BH, Minchenko OH. (2020). Expression of IDE and PITRM1 genes in IRE1 knockdown U87 glioma cells: effect of hypoxia and glucose deprivation. Endocr Reg, 54:183-195.
Publisher | Google Scholor - Minchenko DO, Khita OO, Tsymbal DO, Viletska YM, Sliusar MY, Yefimova YV, Levadna LO, Krasnytska DA, Minchenko OH. (2021a). ERN1 knockdown modifies the impact of glucose and glutamine deprivations on the expression of EDN1 and its receptors in glioma cells. Endocr Reg, 55:72-82.
Publisher | Google Scholor - Minchenko OH, Tsymbal DO, Khita OO, Minchenko DO. (2021b). Inhibition of ERN1 signaling is important for the suppression of tumor growth. Clin Cancer Drugs, 8:27-38.
Publisher | Google Scholor - Minchenko DO, Krasnytska DA, Khita OO, Viletska YM, Rudnytska OV, Kozynkevych HE, Hoian SL, Minchenko OH. (2023). Knockdown of ERN1 modifies the impact of glutamine deprivation on TGIF1, ZEB2, NKX3-1, PRRX1, and SLC1A5 gene expressions in U87 glioblastoma cells. J Endocr Diabetes Res, BioRes Scienti, 1:1-10.
Publisher | Google Scholor - Minchenko OH, Sliusar MY, Khikhlo YP, Halkin OV, Viletska YM, Khita OO, Minchenko DO. (2024a). Knockdown of ERN1 disturbs the expression of phosphoserine aminotransferase 1 and related genes in glioblastoma cells. Arch Biochem Biophys, 759:110104.
Publisher | Google Scholor - Minchenko OH, Sliusar MY, Khita OO, Minchenko DO, Viletska YM, Halkin OV, Levadna LO, Cherednychenko AA, Khikhlo YP. (2024b). Inhibition of signaling protein ERN1 increases the sensitivity of serine synthesis gene expressions to glucose and glutamine deprivations in U87MG glioblastoma cells. Endocr Regul, 58:91-100.
Publisher | Google Scholor - Molina-Ayala MA, Rodriguez-Amador V, Suarez-Sanchez R, Leon-Solis L, Gomez-Zamudio J, Mendoza-Zubieta V, Cruz M, Suarez-Sanchez F. (2022). Expression of obesity- and type-2 diabetes-associated genes in omental adipose tissue of individuals with obesity. Gene, 815:146181.
Publisher | Google Scholor - Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. (2008). Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A, 105:8215-8220.
Publisher | Google Scholor - Riabovol OO, Tsymbal DO, Minchenko DO, Lebid-Biletska KM, Sliusar MY, Rudnytska OV, Minchenko OH. (2019). Effect of glucose deprivation on the expression of genes encoding glucocorticoid receptor and some related factors in ERN1-knockdown U87 glioma cells. Endocr Regul, 53:237-249.
Publisher | Google Scholor - Rubin H. (2019). Deprivation of glutamine in cell culture reveals its potential for treating cancer. Proc Natl Acad Sci U S A, 116:6964-6968.
Publisher | Google Scholor - Rudnytska OV, Khita OO, Minchenko DO, Tsymbal DO, Yefimova YV, Sliusar MY, Minchenko OH. The low doses of SWCNTs exhibit a genotoxic effect on the normal human astrocytes by disrupting the functional integrity of the genome. Curr Res Toxicol, 2:64-71.
Publisher | Google Scholor - Teramoto K, Katoh H. (2019). The cystine/glutamate antiporter xCT is a key regulator of EphA2 S897 phosphorylation under glutamine-limited conditions. Cell Signal, 62:109329.
Publisher | Google Scholor - Tsymbal DO, Minchenko DO, Khita OO, Rudnytska OV, Viletska YM, Lahanovska YO, He Q, Liu K, Minchenko OH. (2020). ERN1 knockdown modifies the effect of glucose deprivation on homeobox gene expressions in U87 glioma cells. Endocr Reg 54:196-206.
Publisher | Google Scholor - van Geldermalsen M, Wang Q, Nagarajah R, Marshall AD, Thoeng A, Gao D, Ritchie W, Feng Y, Bailey CG, Deng N, Harvey K, Beith JM, Selinger CI, O'Toole SA, Rasko JE, Holst J. (2016). ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene, 35:3201-3208.
Publisher | Google Scholor - Wang Y, Zhao S, Zhu L, Zhang Q, Ren Y. (2018). MiR-19a negatively regulated the expression of PTEN and promoted the growth of ovarian cancer cells. Gene, 670:166-173.
Publisher | Google Scholor - Wang VM, Ferreira RMM, Almagro J, Evan T, Legrave N, Zaw Thin M, Frith D, Carvalho J, Barry DJ, Snijders AP, Herbert E, Nye EL, MacRae JI, Behrens A. (2019). CD9 identifies pancreatic cancer stem cells and modulates glutamine metabolism to fuel tumour growth. Nat Cell Biol, 21:1425-1435.
Publisher | Google Scholor - Yoo HC, Yu YC, Sung Y, Han JM. (2020). Glutamine reliance in cell metabolism. Exp Mol Med, 52:1496-1516.
Publisher | Google Scholor - Zhang Y, Li L, Chu F, Wu H, Xiao X, Ye J, Li K. (2024a). Itraconazole inhibits tumor growth via CEBPB-mediated glycolysis in colorectal cancer. Cancer Sci, 115:1154-1169.
Publisher | Google Scholor - Zhang Y, Li Z, Huang Y, Zou B, Xu Y. (2024b). Prognostic significance of cyclin-dependent kinase subunit 2 (CKS2) in malignant tumours: a meta-analysis and bioinformatic analysis. BMJ Open, 14:e073887.
Publisher | Google Scholor - Zhao S, Cai J, Li J, Bao G, Li D, Li Y, Zhai X, Jiang C, Fan L. (2017). Bioinformatic profiling identifies a glutamine-related risk signature for the malignancy of glioma and the survival of patients. Mol Neurobiol, 54:8203-8210.
Publisher | Google Scholor